42 fda structured product labels
Resources for SPL Commercial Software and Conversion Vendors and FDA ... A series of Structured Product Labeling (SPL) data standard training opportunities are being offered to SPL commercial software and conversion vendors as well as FDA-regulated companies which... Comirnaty Not Available - by Warner Mendenhall - CovidLawCast.Com NDCs are listed per FDA Structured Product Label (SPL) document for the BLA licensed product. These codes are not included in CDC Vaccine Code Set files at this time. Pfizer has provided the following statement regarding the COMINARTY branded NDCs and labels:
› industry › fda-data-standards-advisoryStructured Product Labeling Resources | FDA Aug 17, 2022 · The Structured Product Labeling (SPL) is a document markup standard approved by Health Level Seven (HL7) and adopted by FDA as a mechanism for exchanging product and facility information.

Fda structured product labels
HL7 Structured Product Labeling and the True Cost of the Free FDA Web ... The FDA Class I deadline enforcement is occurring on December 8, 2022. Many in the medical device industry make plans to use the free FDA Web Interface based on the assumption that they don't have enough products to warrant the use of Structured Product Labeling (SPL) for their GUDID submissions. OnSIDES, side effects extracted from FDA Structured Product Labels The initial release (v01) of the OnSIDES database of adverse reactions and boxed warnings extracted from the FDA structured product labels. All labels available to download from DailyMed ( ) as of April 2022 were processed in this analysis. Code System Object Identifiers | FDA List of code system object identifiers which are used in Structured Product Labeling (SPL) files which are submitted to FDA. List of code system object identifiers which are used in Structured Product Labeling (SPL) files which are submitted to FDA. ... Food and Drug Administration Product Classification System: 2.16.840.1.113883.6.303: Food ...
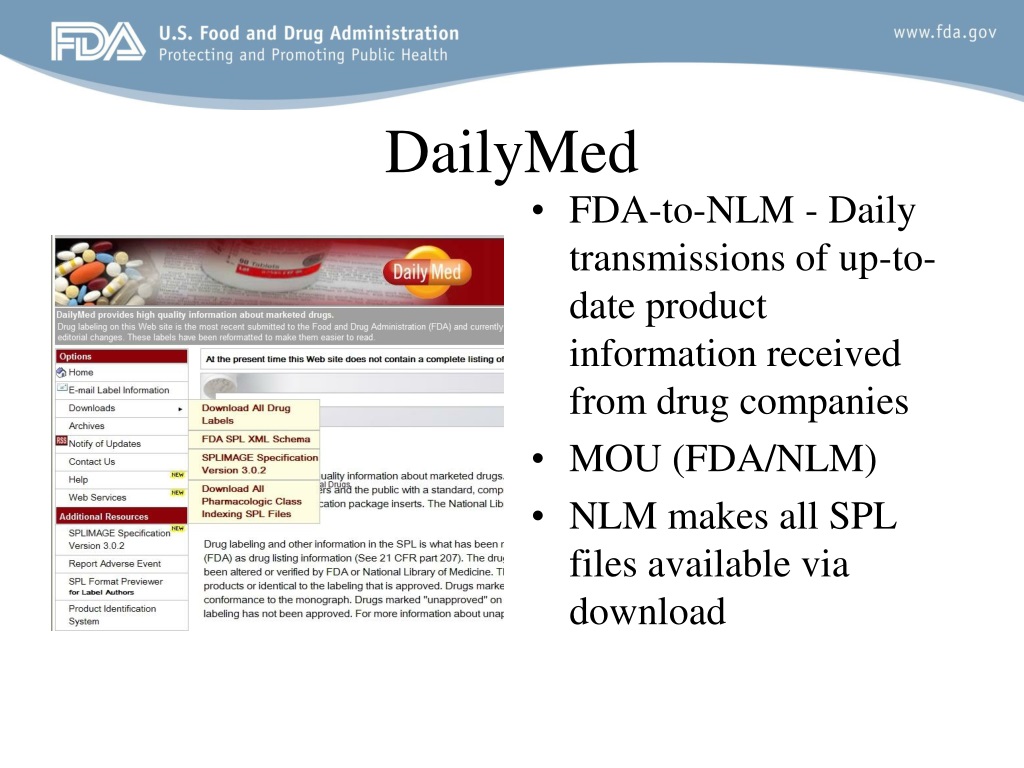
Fda structured product labels. RxNorm Release Notes - United States National Library of Medicine Evusheld is a prophylactic medication for COVID-19 for certain adults and pediatric individuals and contains the antibodies tixagevimab co-packaged with cilgavimab which are administered together. Paxlovid and molnupiravir are oral pills that treat COVID-19 disease. Both can be used in adults. › dailymedDailyMed Sep 15, 2021 · The National Library of Medicine (NLM)’s DailyMed searchable database provides the most recent labeling submitted to the Food and Drug Administration (FDA) by companies and currently in use (i.e., "in use" labeling). DailyMed contains labeling for prescription and nonprescription drugs for human and animal use, and for additional products ... › media › 151710August 23, 2021 Approval Letter - Comirnaty - Food and Drug ... Aug 23, 2021 · Page 3 – STN BL 125742/0 – Elisa Harkins. 10903 New Hampshire Ave. WO71-G112 . Silver Spring, MD 20993-0002 . MANUFACTURING CHANGES . You must submit information to your BLA for our review and ... Risk Evaluation and Mitigation Strategies in Structured Product ... Risk Evaluation and Mitigation Strategies in Structured Product Labeling Format and Additional Pharma Annual Deadlines Life Sciences, Pharmaceutical Regulation, Pharma Manufacturing & Supply Chain, Medical Device, Medical Device Safety and Regulation, Medical Device Manufacturing & Supply Chain Wednesday, September 21, 2022 | 2pm EDT (11am PDT)
NSDE | FDA - U.S. Food and Drug Administration CMS Memo - PDE Editing using the FDA Online Label Repository (PDF) With the exception of the billing unit data in the NSDE document, this file is generated from SPL documents sent to FDA for... dailymed.nlm.nih.gov › dailymed › spl-resources-allDailyMed - Download All Drug Labels Full Releases. Warning: The full human prescription and OTC archive files, dm_spl_release_human_rx.zip and dm_spl_release_human_otc.zip, are no longer available due to size considerations. labels.fda.govFDA Label Search The drug labels and other drug-specific information on this Web site represent the most recent drug listing information companies have submitted to the Food and Drug Administration (FDA). (See 21 CFR part 207.) Over the Counter Medications: FDA Approved Information from OTCLabels.com Over the counter medication labels and full package insert information directly from the approved FDA repository, by OTCLabels.com. ... Our database comes directly from the FDA's central repository of drug labels and package inserts under the Structured Product Labeling standard. OTCLabels.com provides the full over the counter products subset ...
No FDA-Approved COVID-19 Shot Is Available - CatholicCitizens.org The CDC stated that "Comirnaty products are not orderable at this time. NDCs are listed per FDA Structured Product Label (SPL) document for the BLA licensed product. These codes are not included in CDC Vaccine Code Set files at this time. Pfizer has provided the following statement regarding the Cominarty branded NDCs and labels: EOF › media › 161393September 2, 2022 Approval Letter - JYNNEOS - fda.gov Sep 02, 2022 · the final content of labeling (21 CFR 601.14) in Structured Product Labeling (SPL) format via the FDA automated drug registration and listing system, (eLIST) as described at Tech | Best-In-Class Information-Based Solutions and ... Risk Evaluation and Mitigation Strategies in Structured Product Labeling Format & Additional Pharma Annual Deadlines This webinar recording takes a complete look at the annual deadlines that pharmaceutical companies are required to fulfill to become and remain compliant with the US Food and Drug Administration (FDA), with a special focus on the ...
Veterinary Labels: FDA Approved Veterinary Information from VetLabel.com Our database mirrors the FDA's central repository of drug labels and package inserts under the Structured Product Labeling standard. VetLabel.com provides the full animal health subset of the FDA's repository. Veterinary information provided here is not intended as a substitute for direct consultation with a qualified veterinary professional.
RxNorm Overview - United States National Library of Medicine Drugs will first need to be registered in one of the RxNorm data sources, such as FDA Structured Product Labels, to be included in RxNorm. Pharmaceutical companies that want their drugs in RxNorm should submit Structured Product Labeling (SPL) information for each product to the FDA if it has not been done already. Send questions regarding SPL ...
Code System Object Identifiers | FDA List of code system object identifiers which are used in Structured Product Labeling (SPL) files which are submitted to FDA. List of code system object identifiers which are used in Structured Product Labeling (SPL) files which are submitted to FDA. ... Food and Drug Administration Product Classification System: 2.16.840.1.113883.6.303: Food ...
OnSIDES, side effects extracted from FDA Structured Product Labels The initial release (v01) of the OnSIDES database of adverse reactions and boxed warnings extracted from the FDA structured product labels. All labels available to download from DailyMed ( ) as of April 2022 were processed in this analysis.
HL7 Structured Product Labeling and the True Cost of the Free FDA Web ... The FDA Class I deadline enforcement is occurring on December 8, 2022. Many in the medical device industry make plans to use the free FDA Web Interface based on the assumption that they don't have enough products to warrant the use of Structured Product Labeling (SPL) for their GUDID submissions.










.png.aspx)






![PDF] Toward Creating a Gold Standard of Drug Indications from ...](https://d3i71xaburhd42.cloudfront.net/c231e1899261c9d123b8e45ed53703ebbe47f74b/2-Figure1-1.png)

Post a Comment for "42 fda structured product labels"